PIPELINE
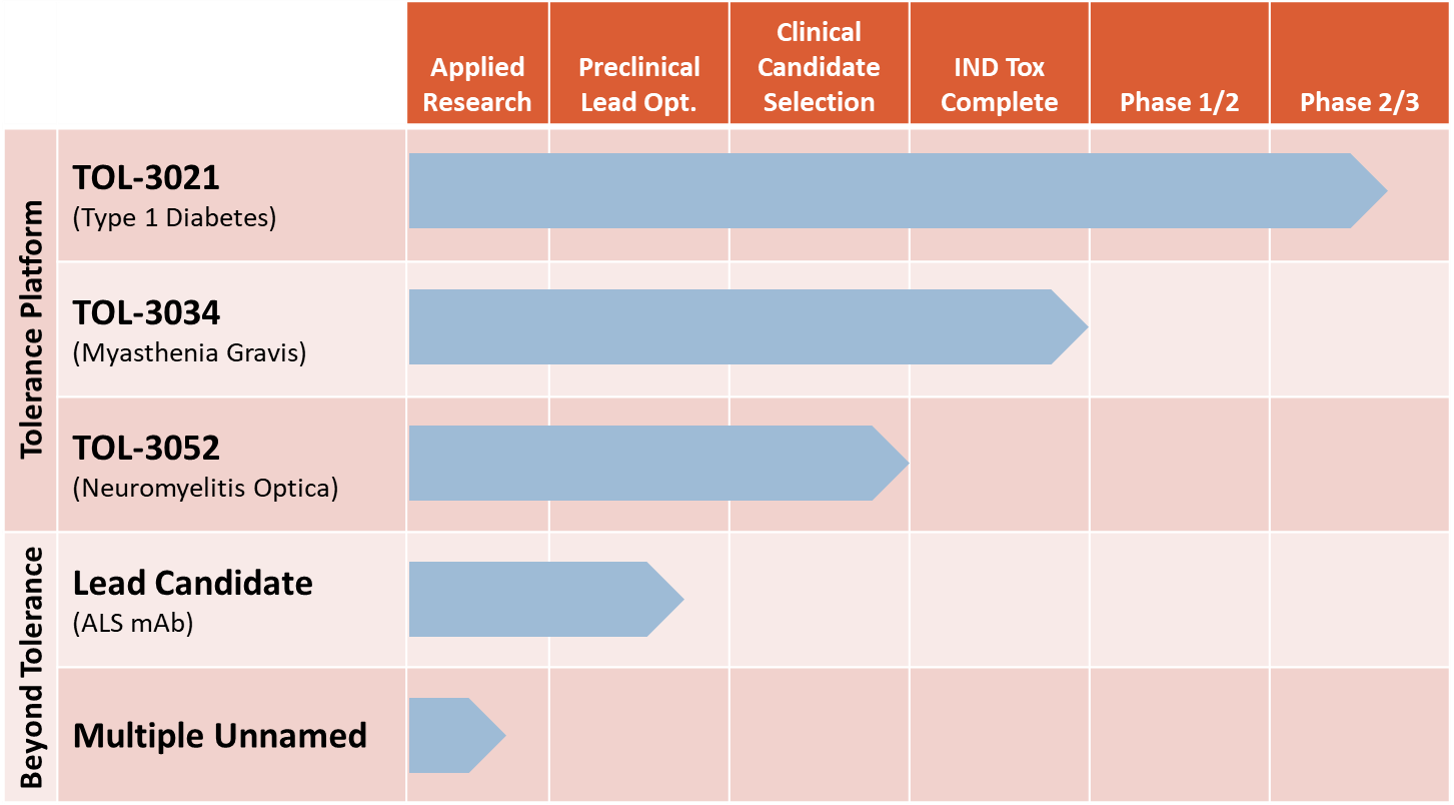
Tolerion Therapeutic Pipeline
Our autoimmune disease product candidates are derived from our proprietary antigen-specific immune tolerance induction platform. We designed our platform to induce tolerance by using a proprietary plasmid that encodes and delivers the disease-specific self-antigen to the immune system.
Plasmids are small circular molecules of double-stranded DNA. Upon injection, the DNA plasmid is taken up by antigen-presenting cells, which are cells that help the immune system determine whether or not an antigen is foreign. Once the plasmid is taken up, these cells express the entire self-antigen protein encoded by the DNA plasmid. The expressed antigen is presented to the immune system in a manner that causes the antigen-presenting cells to turn off the immune cells that are attacking self-antigens and causing tissue injury.
It is through this mechanism that we believe our product candidates selectively reprogram the immune system in a manner that targets disease-causing cells, leaving the remainder of the immune system intact to fulfill its normal function of fighting pathogens and tumors. We believe this specificity will result in improved efficacy with less serious and fewer side effects relative to currently available therapies for autoimmune diseases.
Our most advanced program, TOL-3021, has promising safety and efficacy data from a completed Phase 2a study in patients with type 1 diabetes (T1D). TOL-3034 and TOL-3052 are our clinical development candidates to treat myasthenia gravis and neuromyelitis optica, respectively – two orphan diseases with very well-established causal autoantigens. Having established GMP manufacturing processes for our lead program, we are ready now to initiate CMC development activities for TOL-3034 and TOL-3052 to support first-in-human clinical studies with proof-of-activity endpoints. In addition, we are conducting lead optimization activities to support development of our fourth program, a monoclonal antibody for treatment of ALS. We own all rights to our products in development.

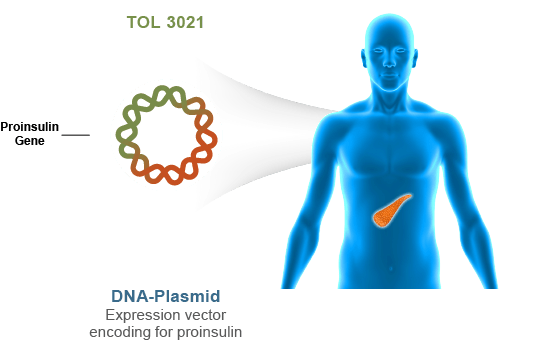
Our Lead Compound: TOL-3021
Tolerion is developing TOL-3021 for antigen-specific immunotherapy of type 1 diabetes (T1D). TOL-3021 has the potential to be the first disease-modifying therapeutic for T1D. TOL-3021 has completed a blinded, placebo-controlled Phase 2a clinical trial which suggests it may preserve or improve pancreatic insulin functionality in T1D patients, thus improving the regulation of blood glucose and mitigating the long-term complications of diabetes. In addition, patients receiving TOL-3021, but not placebo, exhibited a reduction in the frequency of disease-causing cytotoxic immune cells. The frequency of cytotoxic immune cells responsive to other antigens was unaffected by treatment with TOL-3021, suggesting that the drug’s effects are specific to the cohort of immune cells responsible for T1D. These data support the proposed mechanism of action of TOL-3021 and could provide a clinically useful biomarker for assessing treatment success. Based on the promising safety and efficacy data from the Phase 2a study, we have initiated a Phase 2 study in adult patients with established T1D and will soon initiate a pivotal study in adolescent patients with new onset T1D.